The United States is the undisputed, heavy-weight champion of the world when it comes to diabetes. In a report last week by national healthcare insurance giant, UnitedHealth Group, they estimate that more than half of America will be a diabetic or pre-diabetic by the end of the next decade at a cost of $3.35 trillion to our healthcare system if something doesn’t change! Unbeknownst to most of Wall Street, there is a revolutionary new drug weeks from possible approval that will alter the standard of care for tens of millions of diabetics worldwide. Insulin therapy is the most effective treatment for diabetes that exists but there are huge barriers to entry that limit its use. Even with those barriers, insulin is still a $10B+ market worldwide that is growing every year and a major source of revenue for Novo Nordisk, Sanofi-Aventis and Eli Lilly. If approved, Afrezza will be the world’s first Ultra-Rapid Acting Insulin and break down all of the existing barriers to insulin therapy, drastically expanding the existing insulin market.
This article encapsulates the reasons why Afrezza will bring about a fundamental change in the treatment of diabetes. Investors rely too much on analyst comments and published headlines from journalists that perform cursory due diligence at best. Smart investors who look at the underlying technology, the barriers to entry for insulin therapy and the huge unmet need in the market for a better solution will understand why Afrezza is such an advancement above the gold standards that exist today. There is an enormous opportunity in the market to do a better job of controlling this pandemic that Afrezza addresses. It is estimated that 2 new diabetics are diagnosed every 10 seconds worldwide. If Afrezza can make the impact it should, it could become one of the biggest selling drugs of all time. Within this article I’ll cover what diabetes is, the problems with the existing standard of care for treating it including the barriers to entry for early insulin therapy, the facts on Afrezza and how it will bring about a shift to create a new standard of care. In addition, I’ve included rebuttals to the common arguments presented by bear analysts that cover MannKind.
The Structure of Scientific Revolutions, written by Thomas Kuhn in 1962, is a historical look at science and how revolutionary advancements brought about change -- challenging the status quo at the time. He coined the phrase “Paradigm Shift,” describing what happens when the current framework of thought has to be re-written to account for a fundamental shift in an advancement of science. We are about to experience the next Paradigm Shift in the standard of care for treating diabetes but the market simply hasn’t yet realized it.
Insulin is an amazing hormone, produced in the pancreas, which is common among all animals (except a few bugs.) Even insulin produced by most animals would cause a clinical response in a human, including insulin produced from most fish! In a nutshell, insulin is one of the key hormones that tell our body what to do when metabolizing carbohydrates and fats after a meal. It is the central chemical signal for telling the body to take glucose from the blood and transfer it to the body’s cells. After you eat something and glucose or protein from the ingested meal hits the blood, the pancreas releases insulin which regulates glucose absorption into every cell, in every organ system in our body to be used as fuel. When there is too much glucose in the blood (because of the lack of insulin / insulin response) then Hyperglycemia occurs and when there is too much insulin in the body you get low-blood sugar and Hypoglycemia. Hypoglycemia can be mild, just causing you to feel bad but sometimes can be severe, resulting in a coma or even death. Hyperglycemia over-time can produce severe organ damage and it is the measurement of chronic hyperglycemia (too much glucose in the blood) that is used in the principle diagnosis of diabetes.
Glycosylated Hemoglobin (HbA1c) is a form of a metalloprotein in the red blood cells that exists in all vertebrates. It is what is used to quantify just how elevated plasma glucose has been over time, since free plasma glucose levels vary widely, but once HbA1c forms, its levels are relatively constant, and only vary with the formation and death of the red blood cells in which it is found. Measurements of HbA1c are therefore useful in the initial diagnosis of diabetes and in determining dietary compliance among patients. Your HbA1c level is basically an indicator of your average blood glucose concentration for the previous 1-3 months. According to the American Diabetes Association (ADA,) a HbA1c > 6.5 means you have diabetes. If it is between 5.7% - 6.4% then you are considered a pre-diabetic, at severe risk of becoming a Type 2 Diabetic. Drugs used to treat diabetes are evaluated on their ability to lower HbA1c levels.
Diabetes is classified as either Type 1 or Type 2. Type 1 results from either the body’s inability to product insulin, which makes up somewhere between 5-10% of all diabetics. Type 2 Diabetes results from the body’s resistance to insulin, which makes up 90-95% of all diabetes cases. Insulin resistance most commonly affects your muscle and fat cells, which make up 2/3rds of your body. There is no cure for Type 1 diabetes. In the case of Type 2 it is a chronic condition for most and the root cause in 80% of all cases is being overweight or obese, along with genetic predisposition. Through diet and exercise many Type 2’s could cure their disease, but most do not. The CDC estimates there are 56M Pre-diabetics, just a few pounds and a HbA1c point away from being classified as a full-blown diabetic. There are currently close to 28M diabetics in the US or 8% of the population. According to a recent report from the CDC, a child born today has a 1 in 3 chance of becoming a diabetic in a few decades if things don’t change in our country and we could wind up with as many as 500M worldwide at the current rate of expansion. Outside of Obesity, there is no bigger cost to the world’s healthcare system.
Up to 75% of all deaths in Type 2 Diabetic patients are a result of Cardiovascular Disease (CVD.) There is a growing body of evidence that better control of post prandial Glucose (PPG,) can substantially reduce CVD risk. Post prandial Glucose is the amount of glucose in the blood that exists 2 hours after eating a meal. In a study published in 2009, “Postprandial glucose - a potential therapeutic target to reduce cardiovascular mortality” (Peter R, Okoseime OE, Rees A, Owens DR. PMID: 19149642), the authors state, “There is now emerging evidence that postprandial glucose (PPG) contributes significantly to CVD risk, although to date there are no large scale interventional studies underway which test the hypothesis that targeting PPG will reduce CVD risk. Until recently, there was no consensus about the definition of postprandial hyperglycaemia. The International Diabetes Federation (IDF) has now developed new clinical guidelines for postprandial glucose and recommend that 2-hour post prandial glucose levels be kept <7.8 mmol/L. In the last few years more has become known about the cellular mechanisms triggered in response to glucose excursions which may explain this increased susceptibility to CVD. Recently, investigation into the contribution of PPG to HbA1c in subjects with T2DM, has shown that this is maximal in relatively well controlled diabetic subjects. Hence PPG is emerging as a legitimate therapeutic target to minimise CVD risk.” So not only is achieving a HbA1c under 6.5% an important treatment goal with managing diabetes, so is controlling post-prandial glucose levels in order to decrease the greatest risk of death among diabetics. The current standard of care for the treatment of diabetes primarily focuses on HbA1c reduction and not PPG.
To understand the impact of Afrezza, we must first look at the “normal science” for treating diabetes, both Type 1 and Type 2. Now before I go further, I am not a medical doctor or medical professional, just an investor that likes to understand the science behind what I may potentially invest in. My knowledge of diabetes, the treatment of diabetes and Afrezza all come through Internet-based research and conversation with other professionals.
The “Normal Science” of Treating Diabetes:
“Normal Science” is the term phrased by Kuhn to describe the accepted standard prior to the occurrence of a “Paradigm Shift.” There is a current “standard of care” for treating Diabetes which is generally followed by practitioners. For Type 1 patients, they have no choice but to receive Insulin through basal insulin injections and usually at mealtime to cover prandial needs. For Type 2’s, there are a number of options physicians can take to treat the disease but the American Diabetes Association and European Association for the Study of Diabetes has published consensus recommendations for initiating therapy.
In the 2009 study published in Diabetes Care, “Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy -- A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes” (David M. Nathan, MD, John B. Buse, MD, PHD, Mayer B. Davidson, MD, Ele Ferrannini, MD, Rury R. Holman, FRCP, Robert Sherwin, MD, and Bernard Zinman, MD,) the study concludes that “Many patients may be managed effectively with monotherapy; however, the progressive nature of the disease will require the use of combination therapy in many, if not most, patients over time, to achieve and maintain glycemia in the target range.” “Self-monitoring of blood glucose (SMBG) is an important element in adjusting or adding new interventions and, in particular, in titrating insulin doses.” “Mounting evidence suggests that aggressive lowering of glycemia, especially with insulin therapy, in newly diagnosed diabetes can result in sustained remissions, i.e., normoglycemia without need for glucose-lowering medications. Type 2 diabetes is a progressive disease, and patients should be informed that they are likely to require the addition of glucose-lowering medications over time.”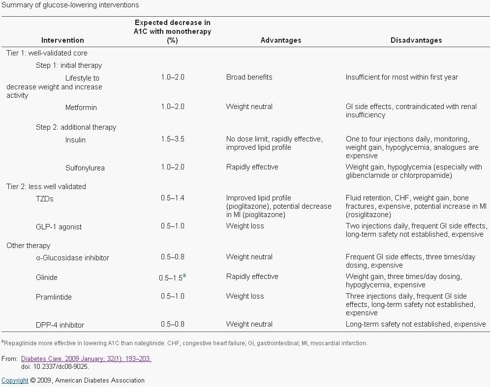
The Barriers to Insulin Therapy
Today, insulin therapy is introduced way too late in the treatment plan. This is a direct result of the disadvantages with the existing therapies, namely 1-4 shots per day, constant self-monitoring of blood glucose, weight gain, risk of hypoglycemia and rapid-acting insulin that is too slow to act and stays in the body too long. In an article published by Irl Hirsch, M.D. (Univ of Washington School of Medicine) on 11/16/2005 entitled “Optimal Initiation of Insulin in Type 2 Diabetes,” Dr. Hirsch writes about the impact insulin therapy can have on Type 2 Diabetics and the optimal initiation plan to use insulin. Dr. Hirsch states that “it can be anticipated that most patients will eventually require insulin therapy to achieve and maintain glycemic control.” Regarding the ADA’s goal of maintaining a HbA1c < 6.5%, “Such stringent levels of glucose control cannot generally be maintained with oral antidiabetic drugs (OADs) alone. However, overall rates of insulin use for type 2 diabetes in the United States are very low. Approximately 11% of patients are treated with a combination of insulin plus OADs and another 16% receive insulin monotherapy. An A1C < 7.0% is only achieved in 36% of patients. Higher rates of glycemic control have been reported among patients with type 2 diabetes who are receiving care from endocrinology practices (61%) and may in part be due to a higher rate of insulin use.”
Dr. Hirsch’s description of the barriers to insulin therapy is spot-on in my opinion, “delays in initiating insulin may stem from both physician and patient barriers. Negative patient perceptions regarding insulin include fear of injections and hypoglycemia. In some cases, patients may perceive insulin as a sign of their personal failure to control the disease. Clinician concerns include hypoglycemia, weight gain, and the misconception that elevated insulin increases cardiovascular risks. In addition, both clinicians and patients may consider insulin therapy to be complicated and labor-intensive.”
The graphic below shows the current recommended treatment plan for diabetics.
Diabetes Treatment Initiation, recommended Standard of Care: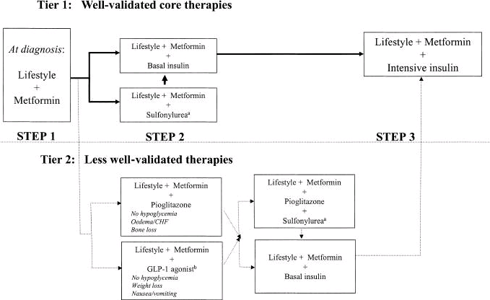
When lifestyle changes and Metformin aren’t enough, the recommended approach is to either try a sulfonylurea or basal insulin next. Physicians generally reach for basal over prandial insulin first because you are only looking at 1 injection per day, rather than 3 in the case of prandial therapy, which patients are less resistant to. Once those aren’t doing the job by themselves, more intensive insulin therapy is recommended, including the use of a prandial (Rapid Acting Analogs - RAA) insulin. The other agents available have their place with some patients but all have their own side effects to consider in relation to their modest reduction in HbA1c and control of post prandial glucose excursions. The earlier introduction of insulin into a patient’s treatment plan is the most effective therapy for controlling HbA1c and PPG but in order for that to happen, insulin must overcome the high barriers preventing wide-spread adoption.
Insulin as a treatment is basically a commodity. Insulin is Insulin is Insulin -- it has been used for treating diabetes for 90 years. The differentiation in the market is around the delivery device and kinetics. Sanofi-Aventis will say that Lantus basal insulin is better than Novo Nordisk’s Levemir basal insulin. Novo Nordisk will say their NovoRapid is better than Eli Lilly’s Humalog Rapid Acting insulin. For the most part, Basal insulin is Basal insulin and Rapid Acting Analogs are Rapid Acting Analogs. They are all injected, have comparable onset of action and remain in the body for similar periods of time. They all have the same barriers to entry for introducing them into the patient’s treatment plan. Think of Ford vs. GM; they both make cars and are competing for the same market share across the same set of consumers, but there is little difference among the vehicles themselves and it simply boils down to personal preference. The same is true for the current mix of insulin providers. This will change with the introduction of Afrezza. It is like bringing a new car to the market that costs the same as the current cars available but the new car can fly, uses half the fuel, is more comfortable and looks better.
The arrival of Afrezza Ultra-Rapid insulin on the market will usher in a new change in the standard of care that I will describe below.
Dispelling myths and supplying factual detail about Exubera and Afrezza
Before moving on to the Afrezza Paradigm Shift, I’d like to clear up the often misconstrued comparison between Exubera and Afrezza. The only common element between both products is that they are both delivered the pulmonary route. Rather than invoke my opinion into the matter, I’ll simply state the facts about each product and let you the reader, draw your own conclusions as to the ability to forecast the market for Afrezza based on the experience from Exubera.
Exubera Facts:
Pfizer’s Exubera was one of the biggest failures in the history of biotech. Originally projected to do over $2B a year in sales, it was a complete flop when introduced and Pfizer took a $2.8B charge to write-off the program. MannKind’s detractors and skeptics continue to point to the dismal sales of Exubera as an indicator for the future of Afrezza. Here are the facts about Exubera, which ultimately led to its failure.
- Exubera offered absolutely no medical benefit whatsoever to Rapid Acting Analogs (RAA.) None.
- Exubera achieved peak insulin levels at 49min compared to injected Rapid-Acting Insulin's 52min (Afrezza peaks at 14min)
- The only benefit of Exubera is that you inhaled it and could prevent mealtime injections, but the kinetics and side effects were similar to regular RAA.
- Exubera had very complex dosing requirements, unique to the product and not linear to standard insulin units. You still had to perform dose titration before a meal and perform Self-Monitoring Glucose Tests.
- Prescribers hated it. It offered no advantage as a therapy, had complex dosing and therefore took a lot of time to explain its use to patients. Patients were often confused by how to use it, which took up a lot of time for physicians and their staff.
- Exubera was priced 20% higher than RAA so Payers didn’t want to cover it.
- The device itself was very inconvenient.
- Despite a popular myth, Exubura did not cause an increase in lung cancer risk.
It is no wonder that Exubera was such a dud in the market given the facts above. The question is why did Pfizer even bother with it? The ‘other’ company’s efforts at inhaled insulin were ceased after Exubera’s failure. Every single one of those programs was a ‘me too’ effort offering no medical benefit, just like Exubera.
Now here are the facts about MannKind’s Afrezza.
Afrezza Facts:
- Afrezza is the world’s first Ultra-Rapid Insulin.
- Afrezza mimics the normal insulin response to a meal that a healthy person would have.
- Afrezza greatly reduces the risk of hypoglycemia
- Afrezza is weight-neutral.
- Afrezza results in lower fasting glucose levels.
- Afrezza inhaler is convenient and easy to use, tested for patients as young as 4 years old.
- Afrezza causes no clinically meaningful impact to pulmonary function (1.7% on the Medtone device and about .5% on the Gen2 inhaler.)
- The Device is disposed of every 2 weeks, eliminating concerns of cleaning or long-term durability.
- Afrezza’s meal-escalation study demonstrated that there was no increased risk of hypoglycemia from Zero up to 150 grams of carbohydrates during a meal. Very little if any need for complex meal titration.
- Afrezza eliminates the need for painful shots, a major concern for new patients requiring insulin therapy and a complaint for those already on insulin therapy.
- Afrezza breaks down all of the existing barriers for introducing insulin therapy to a patient!
In a study published early this year entitled, “Effect of Technosphere Inhaled Insulin on Quality of Life and Treatment Satisfaction” (Mark Peyrot, Richard R. Rubin. Diabetes Technology & Therapeutics. January 2010, 12(1): 49-55. doi:10.1089/dia.2009.0115,) the authors explain that, “research suggests that insulin-naive patients offered an option of taking inhaled insulin are much more likely to initiate insulin therapy than those offered an option of subcutaneous insulin. Thus, availability of the Technosphere insulin system might contribute to reduced delay in the initiation of insulin therapy, a goal suggested by recent clinical guidelines. In addition, research suggests that inhaled insulin is preferred to subcutaneous insulin. Most (85%) patients who received an inhaled insulin during the main phase of a clinical trial chose to continue doing so during the open-label extension phase, and 75% of patients using subcutaneous insulin during the parallel group phase chose to switch to the inhaled insulin.”
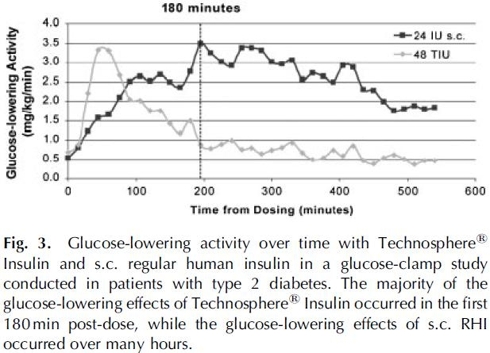
The graph below shows the superior kinetics of Afrezza, practically mimicking a normal person’s insulin response to a meal. This is the key for reducing Hypoglycemia and improving post prandial Glucose levels.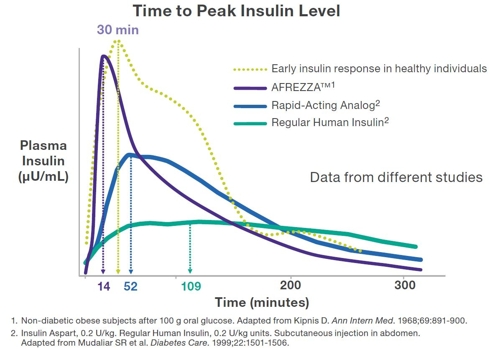
Afrezza greatly reduces the risk of Hypoglycemia compared to standard RAA insulin
Because Afrezza works fast without the long tale that exists with existing prandial insulin options, the risk of Hypoglycemia is greatly reduced. This is clear when the data from the Phase 2/3 trials is pooled and analyzed.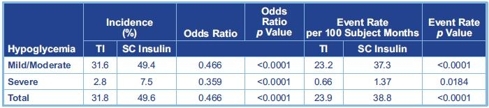
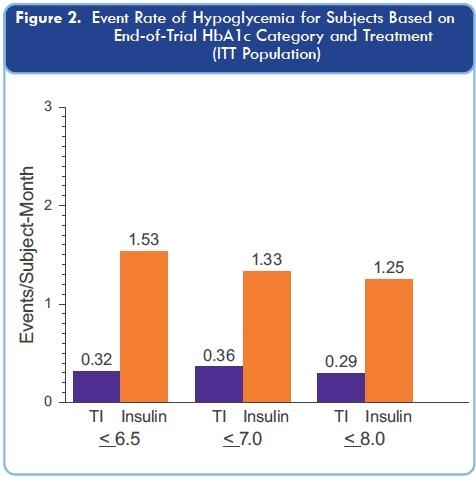
It is too early to make the claim, but one can hypothesize that Afrezza could potentially halt the progression of Type 2 diabetes. Patients Beta-cell function in the Pancreas has often declined by 50% even before they are diagnosed with diabetes. Under the current standard of care, insulin is introduced later in the treatment plan, on average after patients have had a HbA1c level > 7% for 10 years and over HbA1c levels > 8% for 5 years, while the pancreatic function has declined even further during those 15 years. It is theorized that Patient’s β-cell function continues to decline 5% annually.
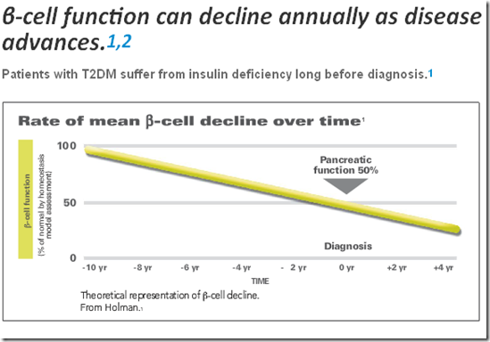
Meal-time insulin control is the first problem that diabetics experience with keeping glucose levels in check. Today, basal insulin is first introduced because it is typically a single daily injection, but it doesn’t adequately control mealtime glucose needs, which continue to stress the pancreas. Through early use of a prandial insulin (instead of basal,) pancreatic stress can be reduced, with the potential to dramatically extend the organ’s lifespan.
The Afrezza “Paradigm Shift” of Treating Diabetes:
Once patients are no longer achieving their therapeutic target HbA1c levels through, lifestyle changes + Meformin, then a 2nd line treatment needs to be added. Under the current standard, RAA insulin isn’t added until after basal insulin has been introduced. The reason for that primarily is that a patient would much prefer to have 1 shot of basal a day then 3 shots of RAA insulin. In a 2008 study published in The Lancet (doi:10.1016/S0140-6736(08)60485-7), “Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial,” the findings were that both basal and prandial insulin were equally as good at controlling HbA1c, but prandial was better at controlling post-prandial glucose excursions throughout the day (now shown to substantially reduce CVD risk) and the conclusion was: “A therapeutic regimen involving the addition of either basal or prandial insulin analogue is equally effective in lowering haemoglobin A1c. We conclude that insulin glargine provides a simple and effective option that is more satisfactory to patients than is lispro for early initiation of insulin therapy, since it was associated with a lower risk of hypoglycaemia, fewer injections, less blood glucose self monitoring, and greater patient satisfaction than was insulin lispro.”These conclusions are consistent with other studies I have read and my own opinion for user acceptance. Now, look at the comparison between Afrezza and existing basal or prandial therapies.
- Reduced risk of hypoglycemia
- No shots
- Greatly reduced need for meal-time blood glucose monitoring
- Little or no dose titration required before a meal
- High satisfaction rating with Afrezza and the device. Given the choice, 75% of patients switched from injected RAA insulin to Afrezza.
- A small survey conducted this summer showed that 95% of medical offices treating diabetes would prescribe Afrezza.
- A large study conducted in the Spring of 2009, demonstrated that physicians would prescribe Afrezza to at least 25% of their patients.
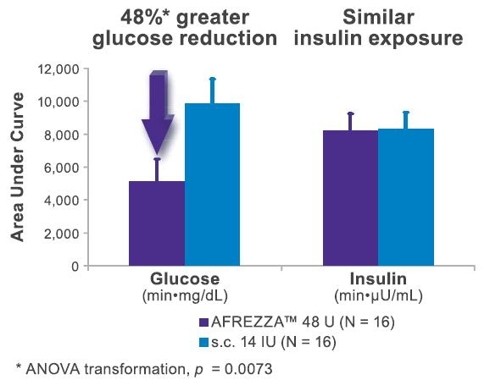
Today, Diabetics on insulin therapy have to maintain a vigorous blood glucose monitoring regime to calculate their mealtime injection dosage requirements and adjust with correcting doses if necessary. For most patients on Afrezza, this will potentially no longer be the case. In a meal escalation study conducted by MannKind and presented as a poster at this year’s ADA, the researchers demonstrated that once a patient is on their optimal dose of Afrezza, just some basic carbohydrate estimates will be needed to ensure their normal dose is adequate. In this study, MannKind tested the effect of eating a meal containing varying levels of carbohydrates with the effect of the post-prandial glucose excursions. This test included eating double the expected amount of carbohydrates, half the expected amount and completely skipping the meal together. All 4 scenarios resulted in PPG Excursions that were well under the recommendations of the ADA, without increased risk of hypoglycemia. This is a complete Paradigm Shift for diabetics on insulin therapy.
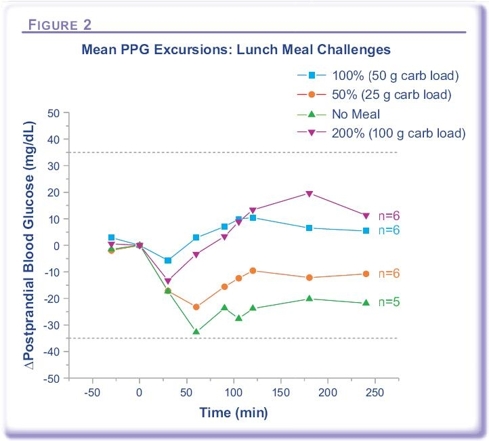
Alfred Mann discussed this study in their conference call held on February 1st, 2010 and had this to say about it: “The objective of this study was to demonstrate conclusively that complex meal titration is not needed. Almost from the beginning of the AFREZZA program, I have expected a very wide therapeutic window for AFREZZA, and especially so in type 2. But I was surprised when the subject(s) had no hypo problems at all, even if they ate absolutely nothing. Let me be more specific. Both prandial excursions for the type 2 patients were within plus or minus 35 milligrams per deciliter, no matter what they ate, even if they ate nothing. The patients all achieved good glycemic control by taking a standard dose of AFREZZA, no matter what they ate and without the risk of hypo. And if there is no need for titration and little risk of hypos, there would be little need for regular prandial glucose [measurement] regimens. We will need to conduct more of these studies in order to demonstrate these findings on a larger scale.”
Afrezza breaks-down the existing barriers to entry for the introduction of Insulin Therapy for both patients and physicians.
Afrezza addresses the primary barriers from patients and physicians for introducing insulin therapy. These are:
- Fear of Injections
- Risk of Hypoglycemia
- Weight Gain
- Complicated and labor intensive
With these barriers broken down, you will see Afrezza rapidly gain market acceptance from patients on existing RAA therapies and patients that should be on insulin but aren’t. Overtime, you will see Afrezza used far earlier in the treatment for Type 2 than you have seen in the past with injected insulin.
The graph below is what may happen over the next few years as Afrezza gains market acceptance.
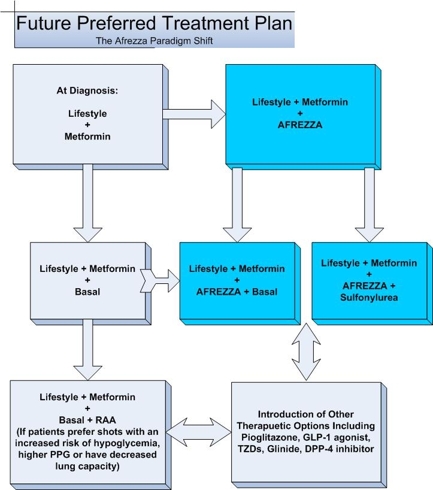
Afrezza opens up a new world for Endocrinologists and Primary Care Physicians to treat Diabetes. Insulin has been the most effective tool at controlling HbA1c but patients have been reluctant to use it for fear of needles and weight gain. Afrezza’s superior absorption kinetics over existing therapies, combined with its ease of use and convenience, will usher in a new era of diabetes management that may have a dramatic impact to the long term health of those suffering from the disease.
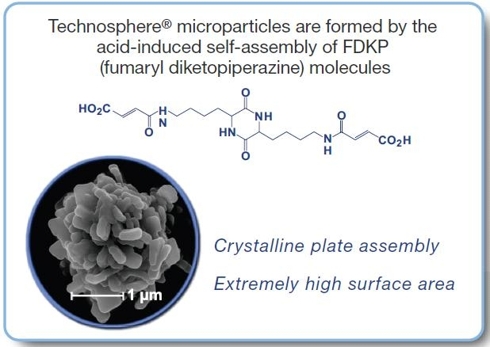
To fully appreciate Afrezza and the value of MannKind, you must understand the unique underlying system that makes Ultra-Rapid Acting insulin possible.
Technosphere is the MannKind proprietary technology that delivers Afrezza and is the crown jewel of the company. It is the platform that serves as a carrier for drugs into the deep lung – whose carrier particles are immediately liquefied allowing the drug to hit the bloodstream almost instantaneously. In fact, the absorption kinetics with Technosphere delivery is just as fast as if you delivered the drug through an injection directly into an artery. The pharmacokinetics of this platform opens up a new world for drug innovation that allows ultra rapid absorption to achieve a desired clinical response. Any small molecule drug, peptide or protein could be delivered via Technosphere particles. In addition to diabetes drugs you could see drugs delivered via Technosphere particles for a broad range of therapies such as cardiovascular disease, migraines, osteoporosis, oncology, fertility and infectious diseases.
Technosphere particles are the perfect size for delivery to the deep lung alveolar tissue. The particles do not build up in the lung and are harmlessly excreted from the body in urine.
For drugs like Afrezza to treat a recurring or chronic condition, you would use a color-coded device for each drug.

MannKind has also developed single-use; disposable inhalers (think vaccines or military applications.) Just push in the button and take a breath. Imagine stockpiling anthrax vaccines in disposable inhalers for immediate distribution in the event of a bio-terrorism attack or members of our military carrying them for quick inoculation in the event of biological warfare.
Technosphere creates a huge competitive advantage not only for Afrezza but for other drugs that will take advantage of the platform. Once Afrezza’s Gen2 inhaler is approved, it will create the precedent for using the same approved platform for delivery of other agents. MannKind already has a mechanism in place to license out the platform to other pharmaceutical companies to take advantage of. You will see more licensing of the technology once the first FDA approval happens utilizing the Technology for Afrezza. Over time, Technosphere could generate hundreds of millions, if not a billion dollars or more in licensing revenue.
Afrezza Valuation:
As I stated in my earlier article “Afrezza’s Market Potential and the Future Value of MannKind” (seekingalpha.com/article/236516-afrezzas-market-potential-and-the-future-value-of-mannkind,) you cannot value Afrezza based on the existing market for Rapid-Acting insulin. I think it is a given that Afrezza will consume the RAA market as evidenced by 75% of patients voluntarily choosing to switch to Afrezza in one of the studies. The key is the earlier adoption of insulin among diabetics AND the migration of Afrezza as the first choice in insulin therapy over basal insulin for use earlier in the treatment plan. This shift magnifies the market exponentially. Afrezza will cost around $2,000 per patient per year, within 5% of existing RAA options. There are approximately 27% of diabetics that are currently on insulin therapy, which equates to about 7.5M patients in the US. As studies show, earlier introduction of insulin therapy is recommended and Afrezza will enable this change. The most recent survey results showed that physicians would prescribe Afrezza to 25% of their patients, which would equal 7M patients just in the US, many of whom most likely aren’t already on insulin therapy.
MannKind’s production capacity for Afrezza in their sole plant will be enough to support 2M patients annually (400K at launch.) At full capacity, that equals $4B in annual Afrezza sales just from this one facility. If Afrezza creates the Paradigm Shift I think it will, they will need several more plants to support worldwide demand. This is a good problem to have if you are MannKind and an investor with a long-term horizon.
The speed by which Afrezza gains market acceptance will be largely determined by the partner that MannKind chooses. I hope the partner is either already a leader in the diabetes field or a big pharma who is hungry to drive a new diabetes portfolio for growth. As a sales person, Afrezza is a dream product to sell. It has clear medical benefits over the competition; it is unique, attractive and has an overall great value proposition for both physicians and patients. With an aggressive sales force and a properly developed marketing campaign, I have no doubt that Afrezza will be a huge success.Fear, Uncertainty and Doubt:
The purpose of this article is to demonstrate why Afrezza will cause a Paradigm Shift in the treatment of diabetes, which I think I’ve clearly shown. I’d like to also address each of the most common arguments that Bears present when it comes to Afrezza. I find it amazing that all of the fear and doubt about Afrezza comes from the financial community and not from practitioners and patients. The most recent marketing surveys clearly show the demand, and the science proves it is a better solution – knocking down all of the existing barriers to entry for introducing insulin therapy. So why do some analysts and journalists continue to forecast an Afrezza failure in the market?
- Exubera Comparison: This one is a tired story that is easily dispelled simply by looking at the facts. I laid out all of the facts above and if you still think Exubera foreshadows the failure of Afrezza, then you probably shouldn’t be investing in biotechnology companies.
- No Advisory Committee: Analysts have backed off this one a bit but some still say the lack of an Advisory Committee means that the FDA won’t approve Afrezza by this PDUFA date. It isn’t surprising at all that an Advisory Committee wasn’t required for Afrezza. There is already precedent for pulmonary delivery of insulin with the approval of Exubera – who had their own advisory committee in 2005 where the expert panel members voted 7-2 in favor of approval. That Advisory Committee was to discuss the efficacy of Exubera and the pulmonary delivery of insulin. Afrezza is a far better product than Exubera for all of the facts described earlier and the FDA knows this. Advisory committees are usually called when there is a Risk vs. Benefit discussion that needs to take place with feedback from outside experts or if the drug under review is a new, novel compound. Afrezza is simple insulin carried on completely inert particles. Afrezza had no serious safety flags through dozens of trials and 5,000 patients that would require a Risk vs. Benefit discussion. There is absolutely nothing with Afrezza that would warrant an Advisory Committee. If one was deemed necessary by the FDA, it would have been called during the initial review in 2009.
- Patient Acceptance: Diabetics are ‘used’ to taking multiple shots a day and won’t want to change. Are you kidding me? This is one of the most asinine statements that I’ve heard on numerous occasions. Now, while this may be true for some, it will be the exception and not the rule. This was even demonstrated in the Quality of Life study referenced earlier, where when given the choice of continuing to use injected RAA insulin or switching to Afrezza, 75% chose to switch. Now think about the millions of diabetics who should be on insulin therapy but resist it. Taking shots is the most widely referenced barrier to entry for beginning insulin therapy. MannKind presented a poster presentation at the 2010 ADA conference to discuss a survey conducted directly to almost 1,100 Type 2 Diabetics regarding the interest of inhaled insulin given the medical benefits laid out in the survey – over 68% of responders said they would be interested in it, and that is before they see commercials on TV or hear about it from their physician. Most of the reasons for the interest expressed by patients were better control of high blood sugar after a meal, avoiding the discomfort and inconvenience of shots along with less weight gain.
- FDA won’t allow MannKind to switch Delivery Devices: In order to switch Delivery Devices, bio-equivalency must be shown between the devices to validate that the previous study data captured still applies with the new device. MannKind conducted extensive studies based on feedback for trial-design from the FDA and recommended assays, which clearly show bio-equivalency between the Medtone and Gen2 devices. Gen2 is just a simpler, more efficient form of the Medtone device. The FDA, despite their recent risk-averse nature, will look at the bio-equivalency data and if it meets their goals of showing bio-equivalency and the device data addresses their concerns which arose with the Medtone device, then they will approve it. Believe it or not, the FDA wants better drugs to hit the market to benefit patients, but they want to ensure they are safe and without undue risk. That is exactly what Afrezza with the Gen2 inhaler is. There is absolutely no reason why any new trials would be needed if bio-equivalency was clearly proven in the studies included with the resubmission. These studies include a Pediatric proof-of-concept study that showed the Gen2 device could be used in children as young as 4 years of age.
Conclusion:
The street has no idea of the profoundly positive impact Afrezza will have in the world of diabetes therapies. Once Afrezza is approved and once the absorption kinetics of it is understood by healthcare providers and patients, Afrezza will become the new “normal science” for treating diabetes. Afrezza will overtake basal insulin as the first course of insulin therapy for type 2 diabetics going onto insulin therapy (earlier in the treatment plan) and will replace existing RAA options for a majority of patients already on prandial insulin. When this happens, not only will Afrezza reach “mega-blockbuster” status but it could become the most valuable drug of our time. When the barriers to insulin therapy come down, the "Paradigm Shift" of Afrezza is inevitable -- improving the health and Quality of Life for millions of diabetics.
Disclosure: Long MNKD