Why I Believe Euphrates Phase 3 Theranostics Trial Of Toraymyxin And EAA From Spectral Diagnostics Will Fail.
Why I believe Euphrates Phase 3 theranostics trial of Toraymyxin and EAA will fail.
Recently I had opportunity to interact with some people on twitter regarding the P3 sepsis trial conducted by Spectral Diagnostics. I presented my views based on data which is publicly available but the bulls seem to be focused on the data published on the company website. I totally respect their right to be long or short any stock, but I beg to differ when they want to cherry pick only select data to prove their point. I apologize if I do the same, but it might be necessary to bring some balance to existing knowledge about the trial. I will try to walk through my points of why I believe the trial will fail. I quickly typed from my notes. Don't jump up and down if there is any typo or grammatical errors.
First I will quickly give an overview of sepsis. Most of this is available online. Wikipedia has some good introductory articles. I will try to provide references where available.
Sepsis is defined as a systemic inflammatory response to infection. Severe sepsis is the syndrome of infection complicated/accompanied acute organ dysfunction [1]. Septic shock is sepsis complicated by a high lactate level or by shock that does not improve after fluid resuscitation.
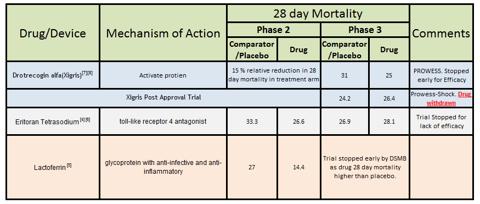
Sepsis is a multi-billion dollar indication pursued by many pharmaceutical companies. There had been several promising drugs in phase 2 studies, but proved to be duds in P3. The only approved drug in US for severe sepsis was Xigris. Very recently the FDA recalled Xigris, by Eli-Lilly. The EMA had ordered a post approval confirmatory trial. The trial had enrolled high risk, severe sepsis patients. The study, called PROWESS-SHOCK, reported a 28-day all-cause mortality rate of 26.4% in patients treated with activated drotrecogin alfa compared with 24.2% in the placebo group of the study, which did not deem the difference statistically significant. The recall came after a decade n billion dollar later [2]. This was not the only recall of a sepsis drug. In 1991 FDA advisory committee recommended approval of HA-1A (marketed as Centoxin in EU), a monoclonal antibody against lipopolysaccharide. The FDA rejected the drug. Later in 1993 a 2nd trial failed to show efficacy for Centoxin and was withdrawn from EU and ROW. A new tool to fight this deadly indication is need of the hour. It always had been. This is the only reason FDA approved Xigris despite half of the panel was against approval [3].
Recent Clinical Trials: I will try to present recent clinical trials which have gone up to Phase 3 and data available. This list may not be exhaustive.
I have not included blood purification methods like that of Cytosorbent Corp or Toraymyxin, because they don't have blinded randomized data.
Now with some introduction out of the way let's go to EAA and Toraymyxin. In simple terms EAA is an assay to measure biologic endotoxins in the blood and the higher the risk higher the EAA. Patients with EAA score greater than 0.6 are supposed to be 3 times more likely to progress to severe sepsis. According to Spectral Diagnostics this might also correlate with higher mortality. Toraymyxin is supposed to remove the endotoxins from the blood. More like hemodialysis. This is supposed to help lower mortality. Now in theory this appears very sound. Identify high risk patients and purify their blood. Brilliant!! So why not all the ICU's in US are lining up for this trial? They should have no problem accruing.
EAA is available in the US since 2003, so why the ICU's are not ordering the EAA at least to identify their high risk sepsis patient. EAA should be used as part of diagnostics in patients with sepsis. So why are they (ICU's) not ordering EAA? Why does Spectral Diagnostics have a market cap of a measly 74 million?
So let's dig a little deeper. The EAA was approved by FDA in 2003. The interesting part is that in 2001 a panel was convened to discuss the utility of the tool for rapid diagnosis of endotoxemia. The minutes of that panel meeting can be found on the FDA website [9]. The panel unanimously voted AGAINST approval. There were many reasons discussed mainly the size of MEDIC Trial, lack of reference, no clinical role and lack of specificity. It had to be a brutal panel. Later with meetings with FDA, spectral agreed to re-classify the device to Class 2 designation and some changes to language in the label. Finally Spectral got the nod to market the test. Now with the FDA hurdle past them, would Spectral be able to market the test, which was unanimously voted down by a panel of US experts, to US doctors? The answer to date is No. As one of the doc puts it "It's quite sensitive to picking up endotoxin, but endotoxin may be present for a variety of reasons in critically ill people. Periods of poor gut perfusion or altered mucosal barrier function, can result in translocation of the endotoxin or gram-negative organisms into the circulation. That could lead to a false-positive EAA result indicating sepsis."[10] So EAA is not the golden standard to identify high risk sepsis patients. It might have some utility but not clear what it is. BioMrieux has a sepsis diagnostic immunoassay tool BRAHMS PCT which was FDA approved and later partnered with Thermo Fisher. Thermo Fisher later partnered this with Roche. This is what would have happened if EAA had any utility.
Moving onto Toraymyxin (PMX B), it is a therapeutic hemoperfusion device that requires a pump capable of circulating at 100 ml/min [11]. The inside of a Toraymyxin column is packed with polystyrene fibers coated with the antibiotic Polymyxin B. This material has a high affinity for endotoxin and as blood passes through the column, endotoxin is tightly adsorbed onto the Polymyxin B coated fibers [11]. Toraymyxin has been marketed in Japan and Europe since 1994 and 2002 respectively [11]. Need to remind the reader here that there was no randomized double blinded trial performed to see if the 28 day mortality of patients in ICU on Toraymyxin vs. a sham control. The ongoing EUPHARATES phase 3 trial is a sham controlled double blinded randomized trial. There are lots of trials, many randomized, with small cohorts of data which show reduced 28 day mortality by Toraymyxin, but none is truly blinded. There are a few worth discussing. First open label randomized trial was carried out in 6 academic centers enrolling 36 patient 17 in Toraymyxin arm and 19 standard of care. In this pilot controlled study [11], the 28 day mortality for PMX was 29% vs. 28% for standard of care. Remind you that this trial was done at premier ICU's across Europe. The lead investigator, Dr.JL Vincent, is one of the leaders in critical care. They concluded that there is no advantage in 28 day mortality but a bigger trial was needed to prove any benefit. Another Large randomized phase 2 (EUPHAS) was launched but exclusively enrolled in Italy. One of the Toraymyxin marketing companies is located in Italy [13]. The trial ran from 2004 to 2007. 64 patients were enrolled 30 in conventional therapy and 34 in PMX arm. The trial was terminated early for efficacy. 28-day mortality was 32% (11/34 patients) in the Polymyxin B group and 53% (16/30 patients) in the conventional therapy group. Though the study shows great efficacy many people including Dr JL Vincent thought that this conclusion was not correct [16]. He points out the statistical analysis and the effect of bias due to un-blinded data. Euphas 2 was launched as a registry to keep track of PMX treated patient in EU. There has been no publication from registry to show if it's in line with the SOC or better. To reiterate, there is no clear data to show that PMX B is more effective in reducing 28 day mortality when compared to SOC in a randomized controlled trial. There have been articles taking pooled data across many small trials and try to conclude that PMX B is better than SOC, but they too feel the need for a controlled trial. The efficacy is more questionable with regards that there is NO data to show that PMX B is better than SOC in North American context. Most of the available data in PMX B is from Italian and Japanese centers. If people think that the efficacy of PMX B is slam-dunk than they need to rethink this.
Very recently the results of failed Eritoran trial were published and the lead author made a comment which I found very interesting. Responding to why an effective endotoxin had failed trial he said it could be too late because the effect of endotoxin occurs within minutes and maybe that's the critical time they were missing [14]. If this statement is validated to be true than it shakes the very foundation of Hemoperfusion of PMX B. Remember that he is also part of DSMB of the ongoing EUPHARATES trial. For him to coin this hypothesis says a lot. Even in the most efficient case it would take around 90 minutes for one cartridge of PMX B. There is a reason that Estor has hard time selling Toraymyxin in Europe.
So now combining a diagnostic tool, which is mediocre at best, and a therapeutic intervention which has no sufficient data in North American context, that too in a very difficult condition what would be your confidence level? I would not like to take chances on this one. I would give it 10% chance of succeeding and then too I would say I am over optimistic in those odds.
FAQs:
Q: Isn't Toraymyxin approved in Japan and EU? It must be safe.
A: Without any large randomized controlled trial it's difficult to say that its safe compared to SOC, especially in a high mortality condition like sever sepsis. I am not sayings it's unsafe or dangerous, all I am saying that more data is needed to prove it conclusively.
Q: Wasn't EUPHAS trial stopped early for overwhelming efficacy of Toraymyxin?
A: Questions are being raised on that and effect of bias in reference [163]. Remember that it was carried out in select Italian ICU's. I can show you one trial [12] where Toraymyxin didn't improve 28 day mortality carried across premiere centers across Europe.
Q: Isn't this an excellent high risk high reward play?
A: If you are comfortable betting 1000 $ to win 10000 $ with about 90 percent chance you WONT win than yes this play is for you. In fact it can even quadruple from current share price of 0.53 before the final data, but be ready for the eventual flush.
Q: The company has said that the combined blinded 28 day mortality is 33.5% this assures them they are enrolling high risk patients i.e. "enriched population", Isn't this good news?
A: What this tells us that the control arm 28 day mortality should be much higher than 33.5% for the trial to succeed. According to the 15 % absolute difference in mortality assumed by the company, the control arm 28 day mortality should be 41.5%. This number is way too high for severe sepsis. When you look at recent articles and try to get a sense of 28 day mortality in severe sepsis, than you get less confident in the current Euphrates trial. Mortality in sepsis is decreasing by about 3% per year in last two decades [15].
References:
- http://www.nejm.org/doi/full/10.1056/NEJMra1208623
- http://gooznews.com/?p=3298
- http://www.thelancet.com/journals/laninf/article/PIIS1473-3099(12)70020-8/fulltext
- Crit Care Med. 2010 Jan;38(1):72-83.
- Crit Care Med. 2013 Mar;41(3):706-16.
- JAMA. 2013 Mar 20;309(11):1154-62.
- N Engl J Med 2001; 344:699-709
- Crit Care Med 2001; 29:2051-2059
- http://www.fda.gov/ohrms/dockets/ac/01/minutes/3795m1-01.pdf
- http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt%7BactionForm.contentReference%7D=cap_today%2Ffeature_stories%2F1107Sepsis.html&_state=maximized&_pageLabel=cntvwr
- http://www.spectraldx.com/toraymyxin.html
- SHOCK, Vol. 23, No. 5, pp. 400-405, 2005
- JAMA. 2009 Jun 17;301(23):2445-52
- news.brown.edu/pressreleases/2013/03/sepsis
- Crit Care Med. 2013 Nov 6.
- JAMA. 2009;302(18):1968-1970