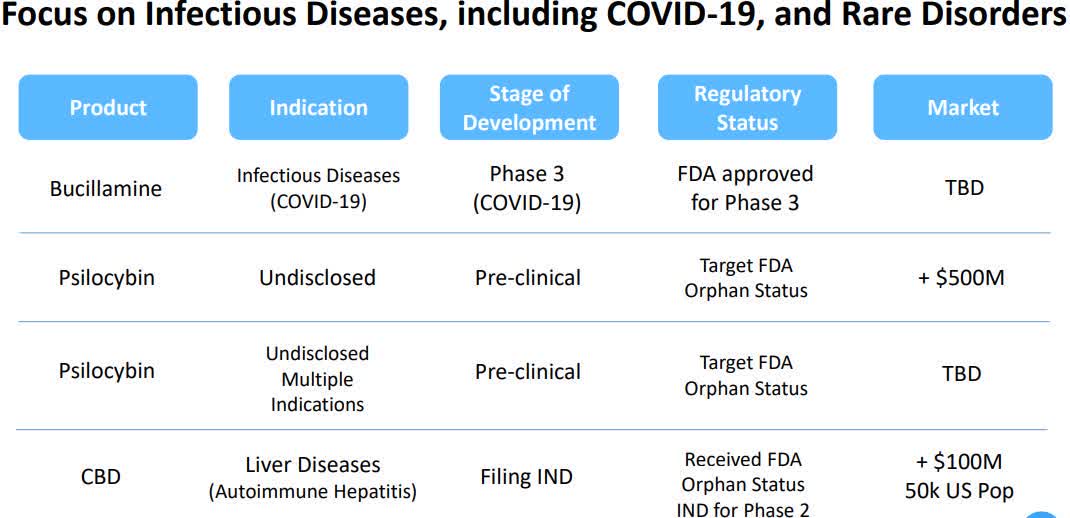
- The independent Institutional Review Board at Advarra, a premier IRB services company in North America, granted approval to Revive Therapeutics' (OTCPK:RVVTF +8.0%) Phase 3 clinical trial protocol to evaluate the safety and efficacy of Bucillamine in patients with mild-moderate COVID-19.
- IRB operates under FDA regulations and is an FDA registered constituted group that has been formally designated to review and monitor biomedical research involving human subjects.
- The Phase 3 confirmatory clinical study will enroll up to 1K patients; primary endpoint being proportion of patients meeting a composite endpoint of hospitalization or death from the time of the first dose through Day 28 following randomization.