Supernus Pharmaceuticals (NASDAQ:SUPN) was founded in 2005 and was previously a subsidiary of Shire PLC (SHPG). SUPN and its management team have developed and commercialized the central nervous system ("CNS") products for more than 25 years. The company's two commercial products are Oxtellar XR® and Trokendi XR® both originally for the treatment of epilepsy. SUPN drugs are extended-release versions of earlier immediate-release branded products. SUPN found that by using extended-release formulations, these two molecules could achieve smoother blood saturation levels resulting in patients with fewer negative side effects and better disease management. In August 2016, SUPN expanded the label for Trokendi by gaining FDA approval for use with migraines. SUPN then built on that label expansion in April 2017 by gaining further approval for Trokendi for use in children over 12 with migraines. This went extremely well for SUPN and led to the strong stock price from 2016 to mid-2018.
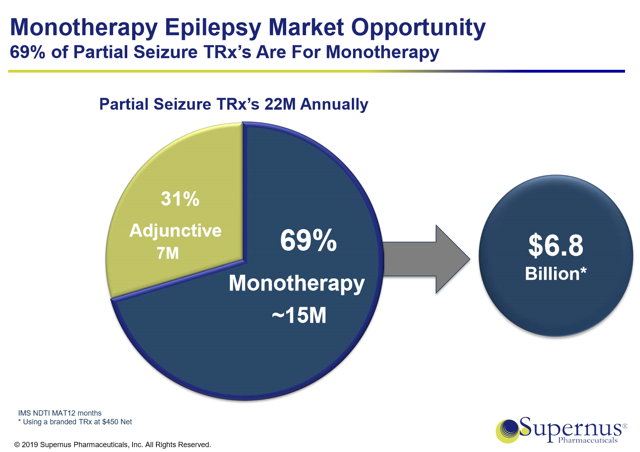
The label expansion playbook that SUPN used with Trokendi has been a big part of the company's success to date. Recently with their drug Oxtellar, SUPN returned to the same strategy by filing an sNDA to expand the drug's label to include its use as monotherapy treatment for partial onset seizures. The product had previously been approved only as an adjunctive treatment for the same indication. The additional market adds roughly 15M new potential patients for Oxtellar. The chart below shows the increase in TAM provided by the monotherapy approval.
(Source: May 2019 Investor Presentation)
To be clear, there are many drugs available for treating partial onset seizures, so we should not expect Oxtellar to gain more than a low single digit percentage peak market share of this expanded opportunity. While this label expansion comes with a very large market, management has cautioned in the past that growth won't be as swift as it