Today, we will study why Immunomedics (IMMU) is an attractive pick in 2020.
Company overview
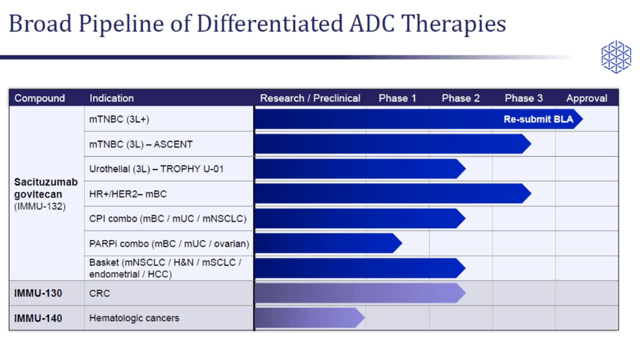
Immunomedics is a clinical-stage biopharmaceutical company focused on developing antibody-drug conjugate therapies for a range of difficult-to-treat cancers. The company is awaiting FDA approval for its lead asset, Sacituzumab govitecan, in third-line plus metastatic TNBC (triple-negative breast cancer) indication. Besides, the company is also studying Sacituzumab govitecan in third-line bladder cancer and ER-positive, HER-2 negative metastatic breast cancer.
Immunomedics is also evaluating Sacituzumab govitecan in combination with checkpoint inhibitors in metastatic breast cancer, metastatic bladder cancer, and metastatic non-small cell lung cancer indications. The company also has two other investigational drugs from its ADC platform.
How Sacituzumab govitecan is proving effective in TNBC
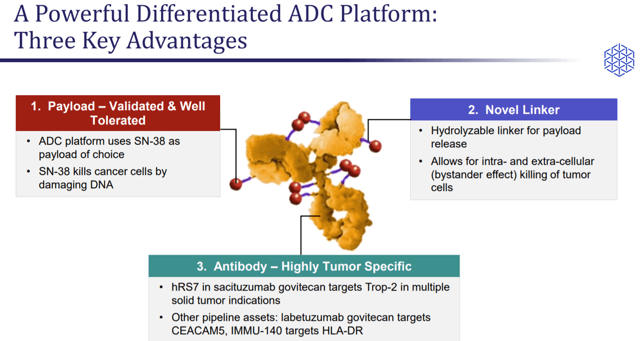
Sacituzumab govitecan is an ADC, which is a drug construct targeting specific proteins or antigens on tumor cells. This drug construct also contains a linked toxin, which is released into the tumor cell or in the tumor microenvironment.
Sacituzumab govitecan targets Trop-2 antigen found on epithelial and solid tumor types. The expression of Trop-2 is usually high in TNBC as well as other types of breast cancer. Sacituzumab govitecan targets this antigen and then delivers the cytotoxin, SN-38, directly in the TNBC tumor and tumor microenvironment. The drug is administered as an intravenous infused product on day one and day eight of a 21-day cycle.
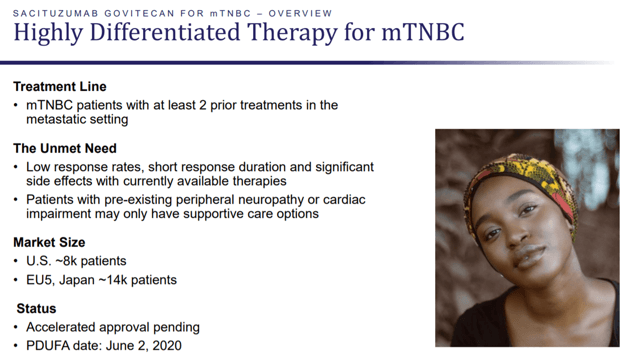
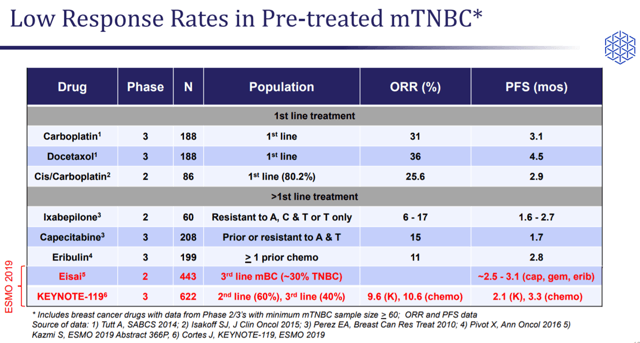
Immunomedics’ most advanced research program involves Sacituzumab govitecan in triple-line plus metastatic TNBC indication. Around 40k patients of TNBC are diagnosed every year across the world. These patients account for 15% of the total breast cancer cases worldwide. Immunomedics is focusing on stage-four metastatic TNBC, which is an aggressive form of breast cancer. There is no standard of care or FDA-approved treatments in this indication.
Estrogen, progesterone, and HER2 are the three types of hormones responsible for the majority