Amarin (NASDAQ:AMRN) had its task cut out for it a couple of years back. I was making a list, and here’s how it looks:
Take the FDA to court on First Amendment rights, and win - CHECK
Successfully complete the REDUCE-IT trial showing Vascepa’s unique ability to reduce cardiovascular risks in at-risk patients - CHECK
Receive FDA approval for this expanded label - CHECK
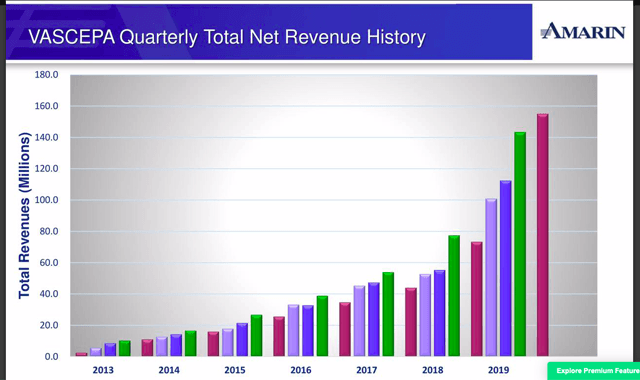
Improve Vascepa sales in a near logarithmic scale every quarter - CHECK, see below
Create a team of expert salesforce to market Vascepa to doctors and patients, thereby proving its ability to not require a partner in the USA - CHECK
Record positive net cash flow from operations - CHECK
Get exhaustive insurance coverage for Vascepa / improve managed care coverage - CHECK
Launch Vascepa ex-US (Canada), and prepare the way for such launch (EU, China) - CHECK
Convince the USPTO to grant them composition of matter patents on EPA - CHECK
Convince medical societies to include Vascepa in their guidelines, and publish in major journals - CHECK
Convince a local judge somewhere in far off Nevada that Vascepa is not fish oil - UNCHECK
This list makes me laugh despite the pain of losing almost 50% of my investment in AMRN in just one night. The entire story sounds so unbelievable. ACP covers a hundred companies a year; we know how this all works. A large number of emerging biopharmaceuticals find it difficult to cross even one of these hurdles. The FDA gets sued all the time - very few lawsuits actually succeed. Companies run Phase 3 trials all the time - very few actually succeed. The success rates of Phase 3 trials and of drug pipelines overall are abysmal - see here for a seminal study and here for a list
If you want to keep yourself on top of the Amarin situation, subscribe to the Total Pharma Tracker. My focus coverage universe is small, but those few stocks that I cover in depth, I go everywhere and see everything - and TPT subscribers read it first.
Read it first on our TotalPharmaTracker mobile app - subscribe today.