Aimmune Therapeutics (AIMT) is a newly commercial-stage biopharma developing a line of drugs designed to desensitize patients with common food allergies. Aimmune's first drug, Palforzia, was approved by the FDA to treat peanut allergies at the end of January 2020 and had just gotten out on the market when the COVID-19 crisis started. While the pandemic will certainly delay Palforzia's ramp-up, I don't see that anything has changed with the company's long-term trajectory. Aimmune is also developing other drugs for egg and tree nut allergies that are in earlier stages of development. In this article, I discuss why I think Aimmune is a compelling value at present.
Palforzia Is A Potential Blockbuster That Will Drive Value Growth Over The Next Few Years
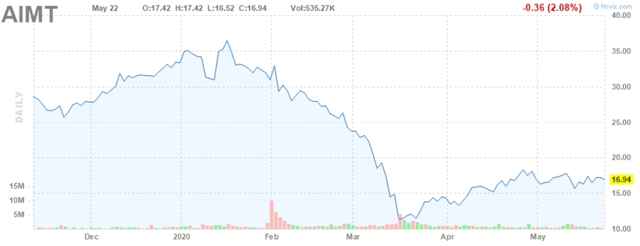
Though the timing of the pandemic was certainly poor for Palforzia's launch, the magnitude of the drop in Aimmune's stock is totally out of proportion to the headwinds created by these delayed sales.
Figure 1: Aimmune Stock Chart (source: finviz)
As you can see from Figure 1, Aimmune has declined over 50% from its recent highs, a drop that is way overdone in my view. Palforzia is a likely blockbuster, and Aimmune currently trades for a little under some of the estimates of Palforzia's peak sales. There is one other therapy for peanut allergy, Viaskin, that is being developed by DBV Technologies (DBVT) that has yet to be approved, but it looks like the lesser of the 2 products to me anyway. Vas Research did a great write-up on the reasons why Palforzia is likely the superior treatment recently that I suggest you check out if you want more detail.
Palforzia has a good jump on Viaskin in getting to market. Although the ramp-up of Palforzia is going slower due to the pandemic, Viaskin doesn't even have a target action