BioMarin (NASDAQ:BMRN) is a leader in rare genetic diseases and when it brings in a product to market, it usually has years of competitive advantage and market exclusivity. However, the FDA’s rejection of its hemophilia A gene therapy in August has resulted in a two-year delay for Roctavian (valoctocogene roxaparvovec) and given its competitor Sangamo (SGMO) a fighting chance to bring its own hemophilia A gene therapy - SB-525 / giroctocogene fitelparvovec - to market at around the same time as Roctavian. So it is imperative to make a study of the clinical differences and similarities between the two assets.
BioMarin’s data
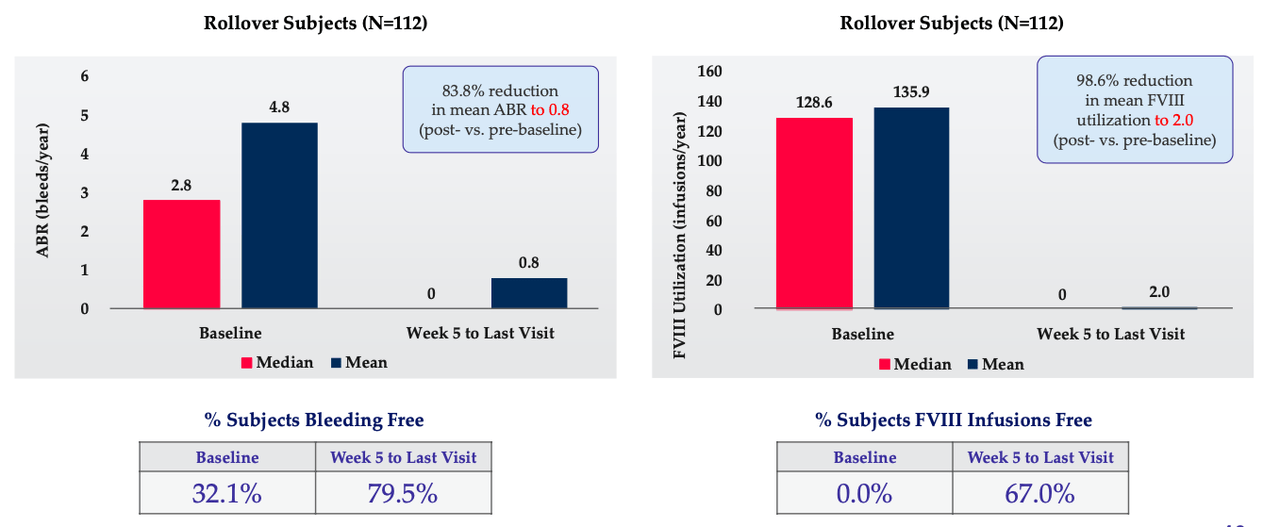
Valoctocogene roxaparvovec (BMN 270) is a gene therapy for severe hemophilia A. A phase 3 study in 132 patients with more than 1 year of data met all primary and secondary endpoints demonstrating superior efficacy to prior Factor VIII prophylaxis agents. The molecule significantly reduced mean annualized bleeding rate or ABR by 84% from 4.8 to 0.8 (n=112) (p-value <0.0001), which is superior to Factor VIII replacement therapy with 99% reduction in mean annualized Factor VIII infusion rate (p-value <0.0001). Factor VIII expression at one year is mean 42.89 iu/dl (n=132). In a subset of patients dosed more than two years ago, a slower rate of decline in Factor VIII expression was observed compared to prior study: mean ABR in this population was 0.9 over these two+ years.
Source - JMP Presentation
In terms of safety, valoctocogene roxaparvovec was well tolerated at a single 6e13 vg/kg dose. No participants developed Factor VIII inhibition, or thromboembolic events. The most common side effects were infusion reactions, ALT elevation, and steroid-related side effects with most resolved during the study.
The company expects to submit an MAA in Europe in the second quarter.
In August, the company received a CRL for the molecule. The
About the author
Thanks for reading. At the Total Pharma Tracker, we offer the following:-

Our Android app and website features a set of tools for DIY investors, including a work-in-progress software where you can enter any ticker and get extensive curated research material.
For investors requiring hands-on support, our in-house experts go through our tools and find the best investible stocks, complete with buy/sell strategies and alerts.
Sign up now for our free trial, request access to our tools, and find out, at no cost to you, what we can do for you.