Jealousy is perhaps the most involuntary of all strong emotions. It steals consciousness, it lies deeper than thought. It is always there, like a blackness in the eye, it discolors the world.” ― Iris Murdoch, The Sea, the Sea
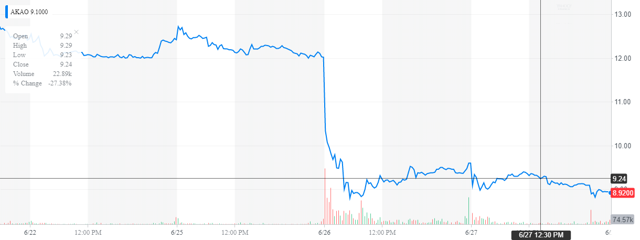
The shares on antibiotic concern Achaogen (AKAO) have surprising dropped just over 25% since trading Tuesday after a FDA decision involving its primary compound plazomicin also known by its brand name Zembri. The government agency approved the antibiotic for use to treat complicated urinary tract infections or cUTI. The FDA declined to approve the drug to treat bloodstream infections, citing lack of effectiveness of the drug in a clinical study.
 A large part of what appears to have doomed Achaogen’s plans for the bloodstream infections indication was its smaller-than-expected sample size that complicated statistical analysis. The company had to amend protocols for the Phase III study because it could only enroll 37 patients out of the planned 286. That resulted in statistical limitations proving Zemdri’s superior efficacy to colistin in the comparator arm. This was a point of discussion at the Ad Comm Panel on the drug in May.
A large part of what appears to have doomed Achaogen’s plans for the bloodstream infections indication was its smaller-than-expected sample size that complicated statistical analysis. The company had to amend protocols for the Phase III study because it could only enroll 37 patients out of the planned 286. That resulted in statistical limitations proving Zemdri’s superior efficacy to colistin in the comparator arm. This was a point of discussion at the Ad Comm Panel on the drug in May.
The reaction in the market today seems overdone for several reasons. First, cUTI is the much longer and more critical indication. Second, this mirrors the Ad Comm Panel recommendation that took place on May 2nd. Zembri as a treatment for cUTI won recommendation in an unanimous 15-0 vote. The same panel voted against recommendation for bloodstream infections by an overwhelming 11-4 vote. Finally, Zembri could still see significant 'off label' use for bloodstream infections. After a follow up meeting with the FDA, a larger trial could start to enroll against that indication and approval might come down the road if study data pans out.
Given all this, it is hard to not see