Editor's note: Seeking Alpha is proud to welcome Georgios Karas as a new contributor. It's easy to become a Seeking Alpha contributor and earn money for your best investment ideas. Active contributors also get free access to SA Essential. Click here to find out more »
On Sept. 12th, 2019, Adverum Biotechnologies (NASDAQ:ADVM) announced preliminary results from the OPTIC-1 Phase 1 trial, a gene therapy trial. As per the company announcement, the phase 1 trial results were positive: "Adverum Biotechnologies Reports Positive 24-Week Data from First Cohort of OPTIC Phase 1 Trial of ADVM-022 Intravitreal Gene Therapy to Treat Wet AMD." The market reaction? The stock tanked with more than 50%. So what happened?
Trial Background and Analysis
An interim analysis of cohort 1 (high dose gene therapy) at 24 weeks was presented. A few select slides will be commented on. Let us walk through the presentation.
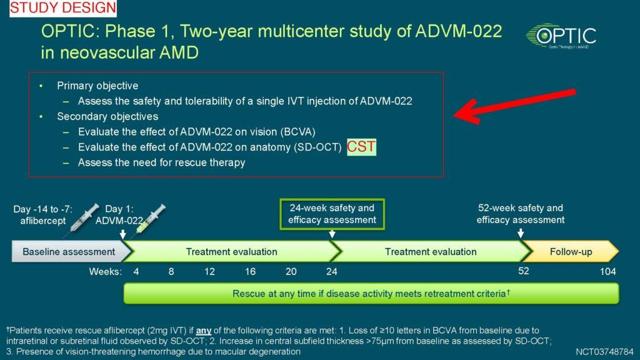
The study is called OPTIC (trial code NCT03748784). It is a phase 1 study, meaning the primary objective is safety and tolerability of ADVM-022 (finding the right dosage for a subsequent phase 2 and phase 3 trial). Per writing of this blog the last trial protocol update was June 4, 2019. After researching the clinical trials archive site regarding study protocol amendments, we could confirm that the dosing regimen was already present in the very first submission. This negates the argument that the company reduced the dosage after the results were known.
The secondary objectives were vision, anatomy and need for rescue therapy. Vision was evaluated with BCVA (higher values are desired), anatomy was evaluated with CST (thickness, lower values are desired) and the need for reduce therapy was a binary yes/no choice (rescue injection with anti-VEGF, the current accepted treatment).
All patients had inflammation post-injection (which reminds us of other trials where inflammation