Argenx is a biotech company based in the Netherlands focusing mainly on oncology and autoimmunity. In Dec 2018, it out-licensed its leading asset, Cusatuzumab (ARGX-110), to Janssen. This partnership offers argenx a one-time payment of over $500M in cash, triggering a shift of focus from oncology to immunology and enabling fast development of multiple autoimmunity pipelines of the company's second largest asset, efgartigimod.
Argenx is a biotech company based in the Netherlands focusing mainly on oncology and autoimmunity. In Dec 2018, it out-licensed its leading asset, Cusatuzumab (ARGX-110), to Janssen. This partnership offers argenx a one-time payment of over $500M in cash, triggering a shift of focus from oncology to immunology and enabling fast development of multiple autoimmunity pipelines of the company's second largest asset, efgartigimod.
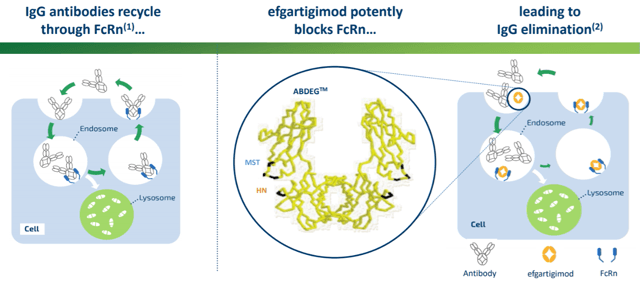
Efgartigimod is a modified Fc region of the immunoglobulin G (IgG) molecule with higher affinity and reduced pH dependence to neonatal Fc receptors (FcRn). Its mechanism of action is straightforward (Fig. 1). Circulating IgG antibodies are constantly recycled in the lysosomes inside the cell through endocytosis. However, neonatal Fc receptors in the endosome bind to IgG and traffic it outward, rescuing them from salvaging in lysosomes. This process maintains the plasma concentration and extends the half-life of IgG, which includes most of the autoantibodies leading to various autoimmune diseases. When efgartigimod is introduced, it out-competes IgG in FcRn binding in the endosome. As a result, a higher portion of IgG is transported in the lysosome and degraded, resulting in a reduction of the overall plasma concentration of IgG, which includes the disease-causing autoantibodies. The reduction of autoantibodies may, in theory, alleviate the symptoms of various autoimmune diseases such as MG and immune thrombocytopenia (ITP).
Figure 1. The mechanism of action of efgartigimod. (Source: Internet)
Given efgartigimod's potential, argenx carefully screened through a list of autoimmune diseases to identify the most economically viable and clinically efficient indications. Currently, the most advanced pipelines are in MG (Fig. 2). In the rest of the article, we are going to mainly focus on this indication.
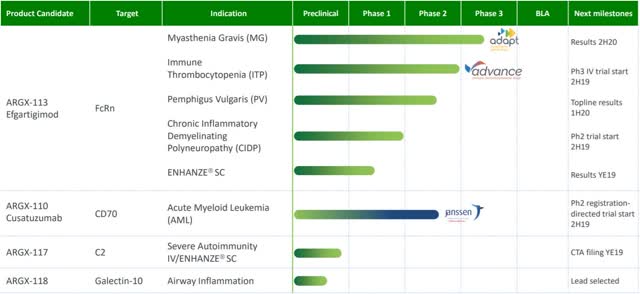 Figure 2. Argenx's current pipelines. (Source: Internet)
Figure 2. Argenx's current pipelines. (Source: Internet)
MG is a rare autoimmune disorder which leads to skeletal muscle weakness and fatigue. It has a prevalence around 150