We have written about how biotech investors should be cautious when companies try to spin failed clinical trials into seemingly positive results through post-hoc analysis and data-mining, and the problems of multiplicity. We even wrote an article about what we believed were Mesoblast’s (NASDAQ: NASDAQ:MESO) data-mined positive results in its failed Phase 2 trial in Chronic Heart Failure (CHF). We believe Mesoblast is performing a similar analysis with its recently failed Phase 3 trial in CHF and that investors should be careful not to get their hopes up for CHF.
We also warn that investors may be making a mistake putting too much weight on the Phase 3 trial in COVID-19-related ARDS, which we believe was recommended to be stopped by the DSMB for futility, or on the Phase 3 trial in chronic lower back pain (CLBP). The announcement of the results of the CLBP trial have been delayed, and we believe it will fail.
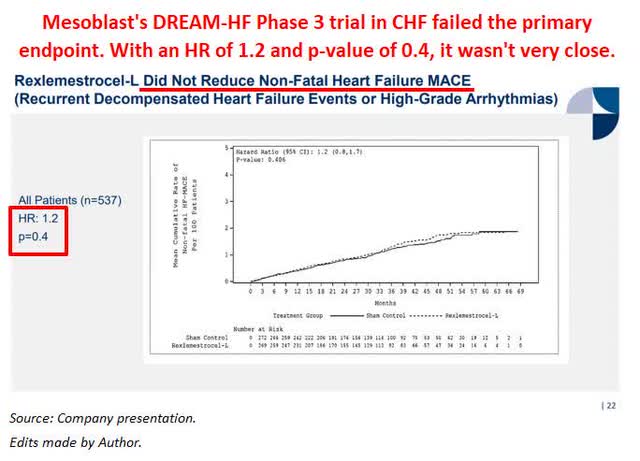
Mesoblast’s Phase 3 DREAM-HF trial in CHF failed its primary endpoint
On January 11, 2021, Mesoblast presented data from the Phase 3 DREAM-HF trial in chronic heart failure for its allogeneic cell therapy rexlemestrocel-L (Revascor). Mesoblast stated that the trial failed its primary endpoint of non-fatal heart failure Major Adverse Cardiac Events (OTCQX:MACE) with a Hazard Ratio of 1.2 and p-value of 0.4.
A HR > 1 for the entire MACE (both ischemic and non-ischemic) means that the HR related to hospitalizations and angioplasty was numerically worse in the Revascor treatment arm. As seen below, not only is there little separation of the curves, but it also appears that the sham control arm (the solid line) performed better in reducing non-fatal heart failure MACE.